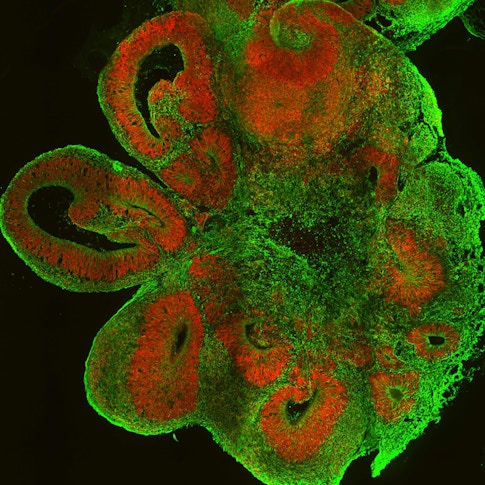
Alterations in the brain associated with autism spectrum disorder (ASD) are thought to arise very early in development, especially during fetal and infant stages (Hazlett et al., Nature, 2017; Willsey et al., Cell, 2013). Analyses of postmortem brain tissue, genomic variations and neuroimaging data have all contributed crucial information to our understanding of human brain development and how it can go awry. However, questions surrounding the earliest periods of brain development in ASD have been difficult to answer in those types of studies. Over the last decade, studies using brain organoids — small self-organizing three-dimensional models of the brain tissue grown in vitro — have been able to recreate at least some of the cellular diversity of the early developing human brain under controlled conditions. Using organoids as an in vitro proxy, researchers can now investigate the differences in molecular and cellular mechanisms that underlie typical and atypical early brain development. In recent noteworthy examples of this approach, two different studies led by SFARI investigators created brain organoids derived from stem cells of people with ASD and neurotypical individuals and identified transcriptional changes that may be associated with ASD during early brain development at the level of single cell types (Jourdon et al., Nat. Neurosci., 2023; Li et al., Nature, 2023).
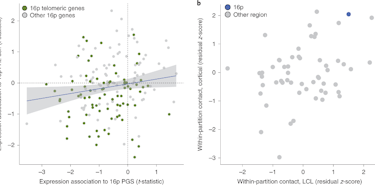
In one of these studies, SFARI Investigators Flora Vaccarino at Yale University and Alexej Abyzov at Mayo Clinic used brain organoids to investigate neurobiological mechanisms linking brain size with idiopathic autism (autism without a known cause) (Jourdon et al., Nat. Neurosci., 2023). This study was supported in part by a SFARI Research Award shared by Abyzov and Vaccarino, and an individual SFARI Research Award to Vaccarino. To create the brain organoids, skin cells from boys with idiopathic ASD and their unaffected fathers were reprogrammed into induced pluripotent stem cells (iPSCs), which were differentiated into forebrain neurons. The researchers then compared gene expression patterns in brain organoids derived from the boys with ASD and controls (their fathers) using single-cell RNA sequencing. In ASD organoids, the researchers reported changes in the activity of certain transcription factors driving cell fate during early cortical development and an atypical imbalance of excitatory neurons, specifically the later-born neurons of the dorsal cortical plate. Interestingly, the direction of these changes in the organoids was correlated with the size of the child’s brain that they were derived from. The boys with ASD who had macrocephaly (an abnormally enlarged head circumference linked to a greater severity of ASD [Lainhart et al., Am. J. Med. Genet. A., 2006]) showed an excess of excitatory cortical neurons, whereas those with ASD who had a normal head circumference (normocephalic) had a deficiency of the same neurons. The findings suggest different mechanisms of ASD pathogenesis in children with ASD who are macrocephalic versus normocephalic.
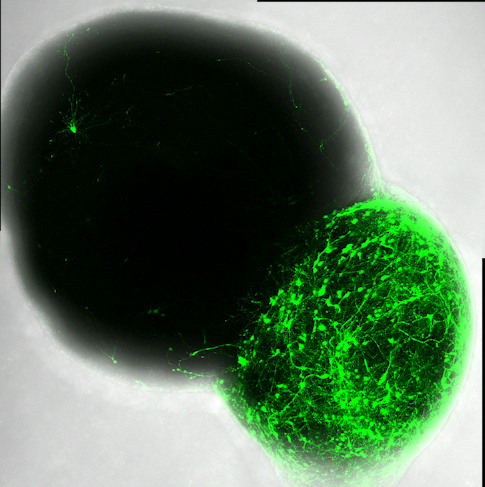
Like Vaccarino and Abyzov, SFARI Investigator Jürgen Knoblich at the Austrian Academy of Sciences also used brain organoids to learn more about the mechanistic role of ASD risk genes in the trajectory of early human brain development in ASD. In their recent study (supported in part by a SFARI Pilot Award), Knoblich and his colleagues developed a unique high-throughput screening approach to study genetic loss-of-function that had not yet been fully explored in brain organoids, in which CRISPR screening technology was combined with single-cell transcriptomics (Li et al., Nature, 2023). They leveraged this approach, which they named the CRISPR–human organoids–single-cell RNA sequencing (CHOOSE) system, to simultaneously perturb a set of 36 high-risk ASD genes involved in transcriptional control in organoids and analyze the effects of the genetic mutations at a single-cell level in dozens of cell types during different stages of brain organoid development. Their findings uncovered critical roles for these ASD risk genes in cell proliferation and survival, as well as cell fate determination, with the most affected cell types being neural progenitors such as intermediate progenitor cells (which generate neurons for all developing cortical layers) and excitatory neurons in the upper layers of the cortex. Among the 36 genes, one of the most significant changes in the composition of cell types resulted from mutation in the ARID1B gene — after perturbing ARID1B, they observed an increased transition of ventral progenitors to early oligodendrocyte precursor cells. They confirmed this finding in brain organoids generated from iPSCs of individuals with ARID1B mutations. Overall, the results showed that, during early brain development, some cell types may be more vulnerable than others to genetic mutations that lead to ASD.
Using brain organoids derived from human pluripotent stem cells, these two studies demonstrate how transcriptional alterations affecting certain cell types early in brain development could contribute to the early emergence of ASD. The studies highlight the increasing usefulness of human brain organoid based assays for understanding the proximal neurobiological mechanisms underlying ASD and other complex human neurodevelopmental syndromes whose origins are not yet fully understood.
Reference(s)
Modeling idiopathic autism in forebrain organoids reveals an imbalance of excitatory cortical neuron subtypes during early neurogenesis.
Jourdon A., Wu F., Mariani J., Capauto D., Norton S., Tomasini L., Amiri A., Suvakov M., Schreiner J.D., Jang Y., Panda A., Nguyen C.K., Cummings E.M., Han G., Powell K., Szekely A., McPartland J. C., Pelphrey K., Chawarska K., Ventola P., Abyzov A., Vaccarino F.
Single-cell brain organoid screening identifies developmental defects in autism.
Li C., Fleck J.S., Martins-Costa C., Burkard T.R., Themann J., Stuempflen M., Peer A.M., Vertesy Á., Littleboy J.B., Esk C., Elling U., Kasprian G., Corsini N.S., Treutlein B., Knoblich J.