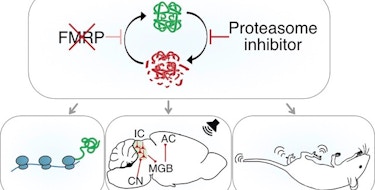
Risk for autism spectrum disorder (ASD) is influenced by several different types of genetic variation. Some individuals with ASD carry genetic variants rarely observed in the general population (for example, rare de novo mutations), while others have variants that occur often enough in the population to be considered common variation. Rare de novo mutations can have large effects in the individuals who carry them; however, the small additive effects of numerous common variants distributed across the genome (i.e., polygenicity) account for much of the genetic risk for ASD at the population level (Gaugler et al., Nat. Genet., 2014). Further investigation is needed to better understand the role of rare and common variation in ASD risk, including the genes and molecular mechanisms that mediate these effects. In a recent study supported in part by a SFARI Research Award, SFARI investigator Elise Robinson and her colleagues used a new statistical approach that identified a large region of the genome — chromosome 16 (16p) — where common and rare variation functionally converge, with relevance for susceptibility to ASD (Weiner et al., Nat. Genet., 2022).
Building on methodology from their previous work on polygenic variation in ASD risk (Weiner et al., Nat. Genet., 2017), Robinson and her colleagues developed and used new statistical methods needed to interpret genomic data from large ASD datasets, in this case, from large ASD cohorts such as the Simons Simplex Collection (SSC), Simons Foundation Powering Autism Research (SPARK) and Psychiatric Genomics Consortium (PGC), as well as genome-wide association data from the Danish iPSYCH consortium. The researchers performed an unbiased genome-wide search for excess over-transmission of ASD’s polygenic influences. They found that the greatest excess of common polygenic risk for ASD is localized to the short arm of chromosome 16p, a 33-megabase region replete with genes expressed specifically in the brain. This region also includes the 16p11.2 locus, which, when duplicated or deleted as a copy number variant (CNV), is a strong genetic risk factor for ASD (Niarchou et al., Transl. Psychiatry, 2019).
In exploring potential overlap in the functional consequences of rare 16p11.2 CNVs and common variation associated with ASD across 16p, the researchers further showed that both rare and common influences are associated with decreased expression of a large group of genes. More specifically, in vitro deletion of the 16p11.2 CNV locus in neuronal cell lines resulted in an average decrease in the expression of brain-expressed genes throughout the 16p region. A similar decrease in cortical gene expression across 16p was seen in postmortem brain samples from donors with an increased autism polygenic risk score constructed with 16p variants. The effects of rare deletion and common variation were correlated at the level of individual genes on 16p, suggesting convergence of rare and common genetic influences on ASD at this region of the genome. The results also showed that 16p has elevated chromatin contact within the region, which might facilitate the transcriptional convergence of rare and common genetic influences. The researchers hypothesized that these transcriptional changes may increase the likelihood of developing ASD.
Overall, this study advances our understanding of the convergent influence of rare and common polygenic variation in ASD risk. The research demonstrates the feasibility of a new approach for obtaining biological insight from ASD-related genomic data across large genomic regions, in contrast to traditional approaches of fine-mapping individual disease-associated variants to the genes through which they act.

Reference(s)
Statistical and functional convergence of common and rare genetic influences on autism at chromosome 16p.
Weiner D.J., Ling E., Erdin S., Tai D.J.C., Yadav R., Grove J., Fu J.M., Nadig A., Carey C.E., Baya N., Bybjerg-Grauholm J., iPSCYH Consortium, ASD Working Group of the Psychiatric Genomics Consortium, ADHD Working Group of the Psychiatric Genomics Consortium, Berretta S., Macosko E.Z., Sebat J., O’Connor L.J., Hougaard D.M., Børglum A.D., Talkowski M., McCarroll S., Robinson E.