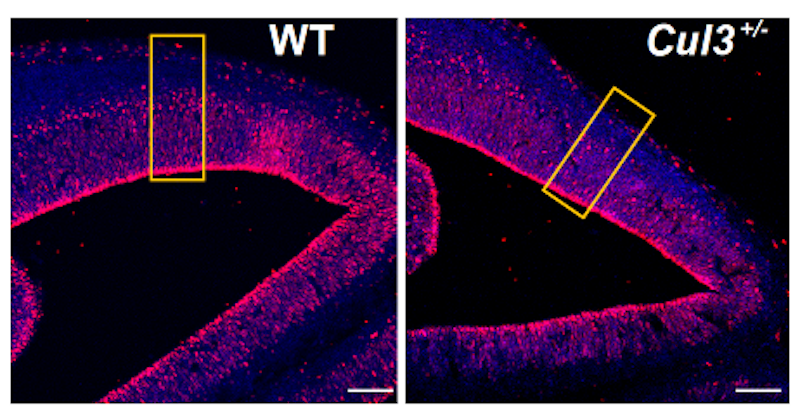
Cullin3 (Cul3) is a high-confidence autism spectrum disorder (ASD) risk gene. A well-characterized ubiquitin ligase, Cul3 directs the breakdown of numerous proteins, affecting many basic cellular functions. SFARI Investigator Lilia Iakoucheva showed that, in mice lacking one copy of Cul3, reductions in Cul3 decrease neurogenesis and alter the neuronal cytoskeleton through an effect on RhoA signaling, likely underlying an observed decrease in brain size (Amar et al., Mol. Psychiatry, 2021). This work was supported in part by a Research Award to Iakoucheva.
Reductions in Cul3 level are associated with decreases in brain volume across many different regions, including the somatosensory cortex, hippocampus and cerebellum. Gene and protein expression profiling, assessments of neuron numbers and morphological analyses in cell culture all suggest that these decreases in brain volume reflect changes in neuronal differentiation and cytoskeletal function that stunt neuronal dendrite growth and reduce neuronal network function.
Cul3 works with a number of adapter proteins in distinct cell types to degrade target proteins. Iakoucheva’s laboratory previously found that Cul3 interacts with the KCTD13 adapter protein in the human fetal brain (Lin et al., Neuron, 2015). In non-brain cells, the Cul3-KCTD13 interaction targets RhoA for degradation (Chen et al., Mol. Cell, 2009), and Iakoucheva’s prior analyses suggested similar targeting might occur in the human brain.
In the brain, RhoA signaling is known to regulate actin cytoskeletal dynamics and neuron growth and migration, all of which are affected in the Cul3 mutant mice. Iakoucheva’s team went on to demonstrate that RhoA cortical expression was increased in Cul3 mice and that pharmacological blockade of RhoA function in neuronal culture from Cul3 mice rescued deficits in dendrite growth and network activity. These findings suggest that there is value in assessing the effects of Cul3 mutations on RhoA signaling in human models such as induced pluripotent stem cells lines (iPSCs) or in postmortem brain samples.
Interestingly, the KCTD13 adapter protein also has a genetic link to neurodevelopmental conditions such as ASD, being located within the 16p11.2 CNV. The dosage of the 16p11.2 CNV is also associated with head and brain size phenotypes in humans; 16p11.2 deletions (decreases) cause macrocephaly while 16p11.2 duplications (increases) cause microcephaly. Intriguingly, RhoA over-activation has also been observed in human organoids produced from the skin fibroblasts of patients with autism by the Iakoucheva lab (Urresti et al., Mol. Psychiatry, 2021). Similar brain changes have also been observed in 16p11.2, Mecp2 and Cntnap2 mouse models. It is therefore intriguing to speculate that RhoA may be a broader mechanistic target of ASD risk genes.
Reference(s)
Autism-linked Cullin3 germline haploinsufficiency impacts cytoskeletal dynamics and cortical neurogenesis through RhoA signaling.
Amar M., Pramod A.B., Yu N.-K., Herrera V.M., Qiu L.R., Moran-Losada P., Zhang P., Trujillo C.A., Ellegood J., Urresti J., Chau K., Diedrich J., Chen J., Gutierrez J., Sebat J., Ramanathan D., Lerch J., Yates J.R. 3rd, Muotri A., Iakoucheva L.


