Genes encoding a great many of the components of the BAF chromatin remodeling complex are known to be mutated in neurodevelopmental conditions, including autism spectrum disorder (ASD). Despite the substantial genetic evidence, the mechanisms by which they confer risk are largely unknown. A new study identifies mutations in ACTL6B as a relatively common cause of ASD, reports on the nature of the phenotype, and reveals an intriguing effect of these mutations on gene expression in resting neurons.
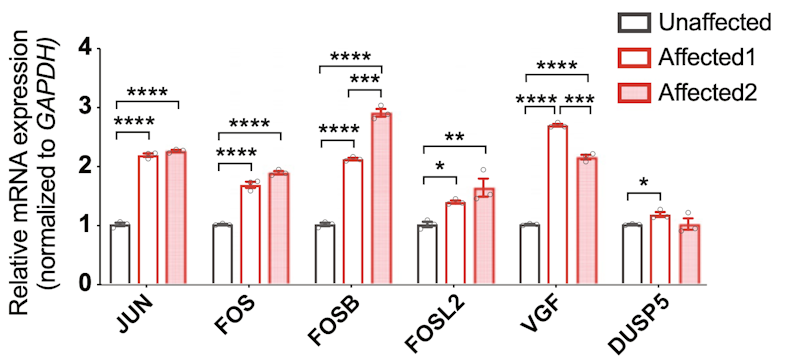
The work was supported in part by a Research Award to SFARI Investigators Gerald Crabtree and Joseph Gleeson. The study builds on a previous Research Award to Gleeson, who established the Simons Recessive Autism Cohort (S-RAC) — a cohort of families with recessive causes of autism identified by exome sequencing. The authors found that mutations in ACTL6B are by far the most common causes of ASD in the S-RAC, and in the new paper, they present data on six such pedigrees. Descriptions of several phenotypes follow, both in humans and in model organisms. These include ‘perfect dendritic retargeting’ in a Drosophila mutant, where dendritic trees in the olfactory system project to the wrong glomerulus with 100 percent expressivity. They further show that ACTL6B mutation in mice and humans results in a reduced or absent corpus callosum, and that mutant mice show deficits in several ASD-relevant behaviors. Finally, Crabtree, Gleeson and colleagues report that a major role of ACTL6B is to suppress the activity-responsive transcriptional program in resting neurons. Data from RNAseq and ATACseq experiments showed a substantial and inappropriate upregulation of so-called ‘immediate-early’ genes in resting neurons carrying biallelic mutations in ACTL6B.
Future work will no doubt involve a survey of activity-responsive transcription in cells carrying mutations in genes encoding other components of the BAF complex that have been implicated in ASD. Such efforts will likely lead to a better understanding of how changes in the timing of transcriptional activity early in neuronal development leads to alterations in downstream circuitry.
Reference(s)
Loss of the neural-specific BAF subunit ACTL6B relieves repression of early response genes and causes recessive autism.
Wenderski W., Wang L., Krokhotin A., Walsh J.J., Li H., Shoji H., Ghosh S., George R.D., Miller E.L., Elias L., Gillespie M.A., Son E.Y., Staahl B.T., Baek S.T., Stanley V., Moncada C., Shipony Z., Linker S.B., Marchetto M.C.N., Gage F.H., Chen D., Sultan T., Zaki M.S., Ranish J.A., Miyakawa T., Luo L., Malenka R., Crabtree G., Gleeson J.


