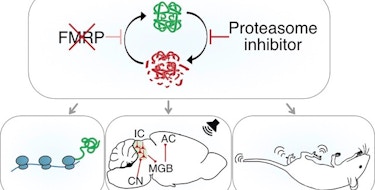
Altered sensory processing is a frequently noted feature of ASD. While some progress has been made in modeling and correcting certain aspects of sensory processing in mice, the distributed nature of the associated circuitry has made this a particularly challenging problem. A new study of a mouse model of Ptchd1-deficiency shows how combinatorial targeting of the relevant neural circuits results in a broader phenotypic rescue. At the same time, another study on PTCHD1 mutations in humans sheds new light on the genetic and biological underpinnings of this particular genetic syndrome.
The new work on mice is supported in part by the Simons Center for the Social Brain at MIT and is based on a previous SFARI Research Award to SFARI Investigators Guoping Feng and Michael Halassa. The authors followed up on their previous findings on mice lacking Ptchd1, which shows enriched expression in the thalamic reticular nucleus (TRN), a key node for automatic sensory filtering. Using an auditory stimulation paradigm, in which responses of neurons of the auditory TRN were recorded with or without increasing levels of broadband noise, they found the Ptchd1 knockout mice to be hypersensitive to noise. While some of the auditory stimulus processing could be restored by administration of a drug that targets SK channels in the TRN, it was only a combination of drugs targeting both the TRN and the prefrontal cortex (PFC) that fully remedied the noise-filtering deficits in the knockout mice. Feng, Halassa and colleagues interpret these results to suggest that the noise hypersensitivity phenotype is modulated both by processes that are intrinsic to the TRN and that are under top-down control from the PFC.
In a separate study supported by another SFARI Research Award, SFARI Investigators Stephen Scherer and James Ellis and colleagues compiled a comprehensive list of 75 reports of mutation at the PTCHD1 locus. They find that many of these mutations actually target a long non-coding RNA (PTCHD1-AS) that flanks the coding PTCHD1 gene, and that the prevalence of autism in individuals with mutations in PTCHD1-AS alone is greater than in those with mutations solely in the coding gene. Induced pluripotent stem cell-derived neurons from individuals with mutations in PTCHD1-AS showed diminished excitatory postsynaptic current frequency. In light of these findings, it will be of interest to determine the expression patterns of PTCHD1-AS in the brain, and whether it has a similar role in sensory processing as the coding PTCHD1 gene.

Reference(s)
Combinatorial targeting of distributed forebrain networks reverses noise hypersensitivity in a model of autism spectrum disorder.
Nakajima M., Schmitt L.I., Feng G., Halassa M.
Synaptic dysfunction in human neurons with autism-associated deletions in PTCHD1-AS.
Ross P.J., Zhang W., Mok R.S.F., Zaslavsky K., Deneault E., D’Abate L., Rodrigues D.C., Yuen R.K.C., Faheem M., Mufteev M., Piekna A., Wei W., Pasceri P., Landa R., Nagy A., Varga B., Salter M.W., Scherer S. W., Ellis J.