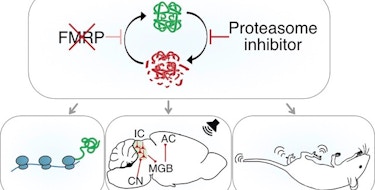
With hundreds of mRNA transcripts identified as direct or indirect targets of FMRP, the protein disrupted in fragile X syndrome, the molecular underpinnings of this condition have proved to be complex. A new study provides additional insight into the roles of FMRP in ribosomal translocation, as well as its downstream effects on levels of a key histone modification that regulates alternative splicing in neurons.
The work was supported in part by a Research Award to SFARI Investigators Kimberly Huber and Joel Richter. By analysis of ribosome translocation in hippocampal slices from both wild-type and FMRP-deficient mice, Huber, Richter and colleagues identified a number of transcripts where the absence of FMRP promoted protein production. One such transcript was that of Setd2, encoding a methyltransferase that catalyzes trimethylation of lysine 36 on histone H3 (H3K36me3). Although the impact of FMRP deficiency on H3K36me3 was complex and variable, changes in its deposition were frequently noted on genes associated with risk of autism spectrum disorder (ASD) (from the SFARI Gene database) and included many genes with roles in presynaptic biology. As H3K36me3 was shown previously to be associated with alternative splicing, the researchers examined such events and found widespread changes in exon skipping. Notably, they reported alterations in exon skipping of microexon 4 in Cpeb4, which has been associated with ASD in humans and with ASD-relevant behaviors in mice (Parras et al., Nature, 2018) .
It’s worth noting another recent study of a related lysine methyltransferase (and ASD risk gene), SETD5, which linked this histone mark with altered splicing due to changes in mRNA elongation dynamics (Sessa et al., Neuron, 2019). Together, these results suggest that a range of disruptions of mRNA metabolism can result in potentially pathogenic changes in splicing.

Reference(s)
FMRP control of ribosome translocation promotes chromatin modifications and alternative splicing of neuronal genes linked to autism.
Shah S., Molinaro G., Liu B., Wang R., Huber K., Richter J.