
SFARI’s Bridge to Independence (BTI) Award program provides funds and career mentoring to early-career scientists (e.g., post-doctoral fellows) to aid them in the transition to independent academic research positions. Launched in 2015, the BTI program currently comprises 31 fellows. In order to provide support systems and community, BTI fellows also meet annually to present their ongoing work and to discuss career and research hurdles and solutions. The 2020 BTI virtual retreat had BTI fellows participating in break-out sessions, where they could talk with mentors and each other to discuss practical skills for becoming successful principal investigators.
Aakanksha Singhvi was the recipient of a BTI award in 2016 while she was a postdoctoral fellow with Shai Shaham at Rockefeller University. In 2018, Singhvi became an assistant faculty member at the Fred Hutchinson Cancer Research Center and an affiliate assistant professor in the Department of Biological Structure, University of Washington School of Medicine. Singhvi’s research program focuses on the mechanistic roles that glia play in the nervous system, using C. elegans as a model organism to examine how glia and neurons communicate to control perception and behavior and to understand how changes in glial-neuronal communication may lead to disease states.
I recently spoke with Singhvi about the work her laboratory is doing and how the BTI Award aided in her transition to an independent faculty position.
The interview has been edited for clarity and brevity.
Tell me about your career path and the work that is ongoing in your laboratory.
I study how glial cells work in the brain. During my Ph.D., I was studying a process called asymmetric cell division that gives rise to all the different types of cells in the brain. I wanted to know how different kinds of neurons are born and how they become different from each other. The same kind of biology also makes a second group of cells in our brain called glia. Essentially, half of the cells of your brain are these glial cells, but we know very little about what they do. That really piqued my interest. How can we understand how the brain works, and mechanisms that lead to conditions like autism, if we don’t understand half of what the brain does?
I became fascinated thinking about mechanistic ways in which these glial cells talk to neurons, how they are impacting what the neurons are doing or not doing, and how they affect how we sense the world and make memories. Having done C. elegans biology before, it seemed like worms could be a very powerful platform to start thinking about these types of questions. So I came to New York and started my postdoc with Shai Shaham because, at the time, his lab was starting to study glial cells in C. elegans systematically. While I was in Shaham’s lab, I helped validate C. elegans as a platform to study how glial cells communicate with neurons, how they affect how an animal senses its environment, and how they affect animal behavior. That interest has really been the focus of my research ever since.
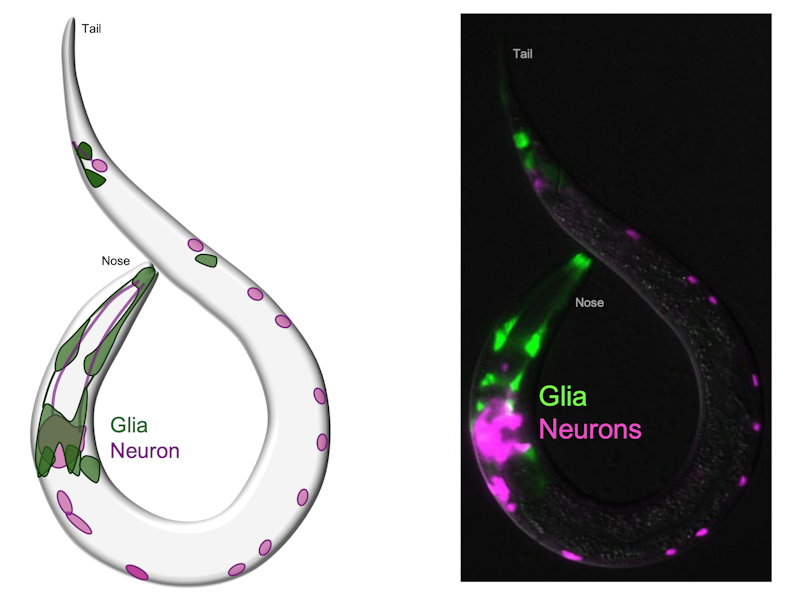
You mentioned wanting to use C. elegans as a model system to look at glial mechanisms. What are the advantages that C. elegans offers to address such research questions?
What’s powerful about C. elegans are its features which give us a unique ability to study each glial cell with single-cell and genetic detail. Importantly for my work on glial-neuronal communication, C. elegans has what’s called a deterministic lineage, which means we can track the development of every cell in the organism and know exactly how each cell is born and develops. This process is so stable that we can actually give a name to each cell that is formed. We know exactly which neuron each glial cell is talking to, and that glia-neuron pair is the same across every single animal. So you can make a population of transgenic animals, but focus on a single conversation between a single glia-neuron pair with this kind of exquisite cellular specificity. Also, because the animal is optically transparent, you can physically look at that single glia cell inside a living animal with all kinds of microscopy, including super-resolution microscopy, that we use routinely. You can also start doing mechanistic studies or genetic studies at that level, and you are doing all of this in the context of a connectome that’s already mapped out.
I like that this means that a lot of our studies are in vivo in a behaving animal, and we can zoom in or out across different focus levels. You can use genetics to manipulate individual cells at will, you can ask how this impacts single cell biology, and you can also test how the same manipulation changes the animal’s sensory behaviors and other behaviors like eating food, mating, sleeping, and memory.
“I realized that almost every glial molecule that controls neuron shape, including the one I had identified, seemed to be implicated in autism.”
What got you interested in autism research, and where is that research trajectory now going in your laboratory?
My connection with autism work, and SFARI, really started with a couple of discoveries towards the end of my postdoc. The first came from the genetic screens we were doing in C. elegans. One of the molecules we identified, called KCC-31, was implicated in autism. In reading papers for background literature, I realized that almost every glial molecule that controls neuron shape, including the one I had identified, seemed to be implicated in autism. So that really got me curious.
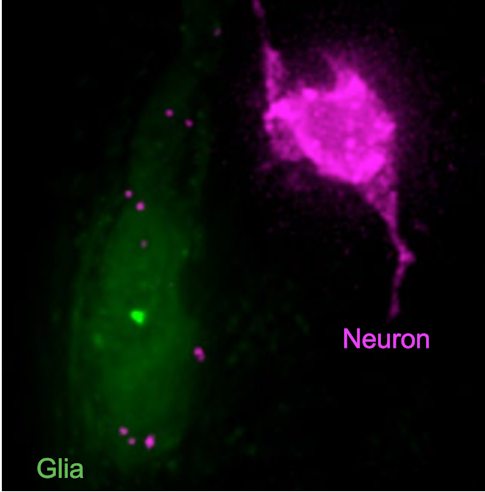
The second thing that got me interested in autism research were some of the first discoveries in my own lab, where we found that glial cells in the peripheral nervous system are actually eating bits and pieces of sensory neurons, and that this response can shape the animal’s sensory responses2, 3. Such pruning functions of glia have been suggested to play a role in autism, but we still understand very little about this process. All of this got me to thinking that while changes in glial-neuronal interactions are being suggested in autism, and other disorders too, it isn’t clear how glia actually contribute to the condition. And that, if we are to figure out how glial dysfunction affects disease states, we need to know how glia work normally.
Our discoveries provide us with a first layer of molecular mechanism. And now we can use the powerful genetic toolkits at our disposal in C. elegans to build off of that initial discovery to further explore molecular mechanisms of glial-neuronal communication in greater depth.
You were one of the earlier recipients of the SFARI BTI Award, having become a fellow in 2016. How did this award help with your successful transition to becoming a principal investigator and running your own lab?
I received the BTI Award offer while I was on my job interviews, and it certainly was a nice confidence-builder and a validation of the science I was planning to pursue. In practical terms, the BTI Award has also been a huge support financially, scientifically and as a community builder.
Financially, I felt like I could hit the ground running. I hired people right off the bat. Instead of stressing out from day one about managing start-up funding, the BTI support gave me the luxury to be even more ambitious about my science program, and just focus on getting things off the ground quickly. That really was a big plus.
“[…] the support from the BTI program, from SFARI and from all the senior advisors have positively influenced me on so many levels as I set up my lab. ”
I think the award also expanded my scientific world-view. I did not start as an autism researcher, but began thinking about autism because I kept finding intriguing links between my research and autism cropping up. Coming to the SFARI meetings, interacting with autism researchers and hearing their findings from diverse perspectives spanning computational to clinical neuroscience, has really helped me go deep quickly. This has absolutely shaped how I see my work in the context of autism, and vice versa. It is an exciting new dimension to my lab’s science, and an avenue I plan to continue beyond the BTI fellowship.
Finally, having the BTI fellows community has been invaluable, and I hope we stay in touch as alumni. Their kinship, the support from the BTI program, from SFARI and from all the senior advisors have positively influenced me on so many levels as I set up my lab.
The BTI Award program offers mentoring and sponsors an annual retreat for BTI awardees—with the goal of aiding the transition from a post-doctoral position to running your own lab. What are your thoughts on these BTI support systems?
This year, SFARI hosted mentoring round table discussions as part of the BTI retreat. We would have a senior investigator and five or six of us BTI fellows in a breakout room. Each group would have an open discussion on one topic, whether it was grant management or personnel management, or what have you. I think the topics were very well chosen and very relevant. This idea was fabulous, and I hope they continue these discussions going forward. In my view, these mentoring discussions would be very helpful for starting faculty such as the next rounds of BTI awardees.
Starting your own laboratory is a challenging endeavor in any year, but this past year has been particularly tough in light of the COVID-19 pandemic. What impact have the constraints resulting from the ongoing pandemic had on your career and those of your junior colleagues? And are there ways in which you think that SFARI resources and the SFARI community could help mitigate some of these affects?
The past year has been crazy. I feel like the lab was up and running, and everything was going fantastic. And just as we were finally getting to cruising altitude as a new lab, we had to shut down. Whether it is work or morale, this year has been challenging for all of us, and with a serious loss to productivity. As a junior lab, we did not have a backlog of papers to finish writing, or data to catch up on analyzing. So, one thing I worried about during the lockdown was that I did not get to maximize my own plans from the SFARI BTI support. Our lab work was compromised for effectively 30 percent of our three-year BTI funding lifetime. Of course, that has been true for everyone, but for junior faculty, these first few years are where we are establishing productivity benchmarks. So, if there is way to help extend that support by a year or so, I think that would be a huge help to junior faculty. Having said that, I also realize that this is no small challenge for any funding agency.
What suggestions do you have for additional BTI-supported efforts, particularly in the context of supporting underrepresented groups and increasing diversity in neuroscience?
One thing I am thinking of more as I build my lab is mentorship, particularly with a view on diversity and the different considerations we ought to be aware of for different trainees. One of my students was awarded a Howard Hughes Medical Institute (HHMI) Gilliam fellowship last year. This award is given to a faculty-graduate student pair and, as part of this award, HHMI has hosted a series of formal sessions on mentoring for the faculty awardee cohort. I’ve found these sessions to be very useful, just as a place to discuss and listen.
Most of the SFARI Investigators I know are deeply vested in their trainees and take their mentorship roles seriously. So, I think it could be a great opportunity to explore having similarly structured conversations through SFARI, so we can all learn from each other’s experiences.
References
- SFARI 2020 Bridge to Independence Award fellows announced
- SFARI Bridge to Independence program: Looking back, looking forward
- SFARI Bridge to Independence fellows discuss new research findings and career challenges at virtual retreat
- A Conversation with SFARI Bridge to Independence Investigator Seth Shipman


