
Seth Shipman‘s laboratory focuses on understanding the molecular and cellular mechanisms shaping brain development, with the aim to better understand the biological processes leading to neurodevelopmental disorders such as autism spectrum disorder (ASD). His research has also been instrumental to the development of a CRISPR-based technology, called ‘molecular recordings,’ which allows storing, tracking and retrieving biological information within live cells.
In December 2017, while a postdoctoral fellow in the laboratories of George Church and Jeffrey Macklis at Harvard Medical School, Shipman was selected as an awardee for a SFARI Bridge to Independence Award. SFARI established this grant program with the goal of investing in the next generation of top autism investigators by facilitating their transition to research independence and providing grant funding at the start of their professorships.
Now an assistant investigator at the Gladstone Institutes and an assistant professor at University of California, San Francisco, Shipman is using his Bridge to Independence Award to apply the molecular recording technology to study how different cell types arise in the brain during normal development as well as the implications for disorders where brain development goes awry, such as in ASD.
I recently spoke with Shipman about the work his laboratory is doing and how the Bridge to Independence Award aided his transition to an independent faculty position and to focus his research on autism. The interview has been edited for clarity and brevity.
What was the research focus of your postdoc?
I went to Harvard Medical School with the idea of trying to better understand early events in the formation of the brain. I wanted to understand how different cell types arise. Specifically, I wanted to know how the directions to make these cell types are programmed into our genome and executed over the time course of brain development. We’re actually pretty good at understanding what’s going on in a biological system at a moment in time; for instance, we can take a snapshot and look at what the cells look like, the proteins that are present in those cells and the genes that are being expressed. But to understand what’s happening over long periods of time in the developing brain is problematic because the kind of technologies that give us good access to snapshots of biology don’t work so well when you add a time component.
“What if the data didn’t have to be collected by a scientist, but rather the cells could collect the data for us?”
So, we tried to get around this basic problem. I realized that if I could gather data from live cells, then I wouldn’t have to worry about these snapshots, because the data would be logged as you go. But the only way that people do that is by watching the cells through a microscope, with a marker that you follow over time. That setup is not really compatible with the developing brain. And so, instead, I took a leap and said, “Well, what if the data didn’t have to be collected by a scientist, but rather the cells could collect the data for us? What if the cells could actually log information internally?” And that turned into what we call the ‘molecular recorder’1,2.
What is the molecular recorder?
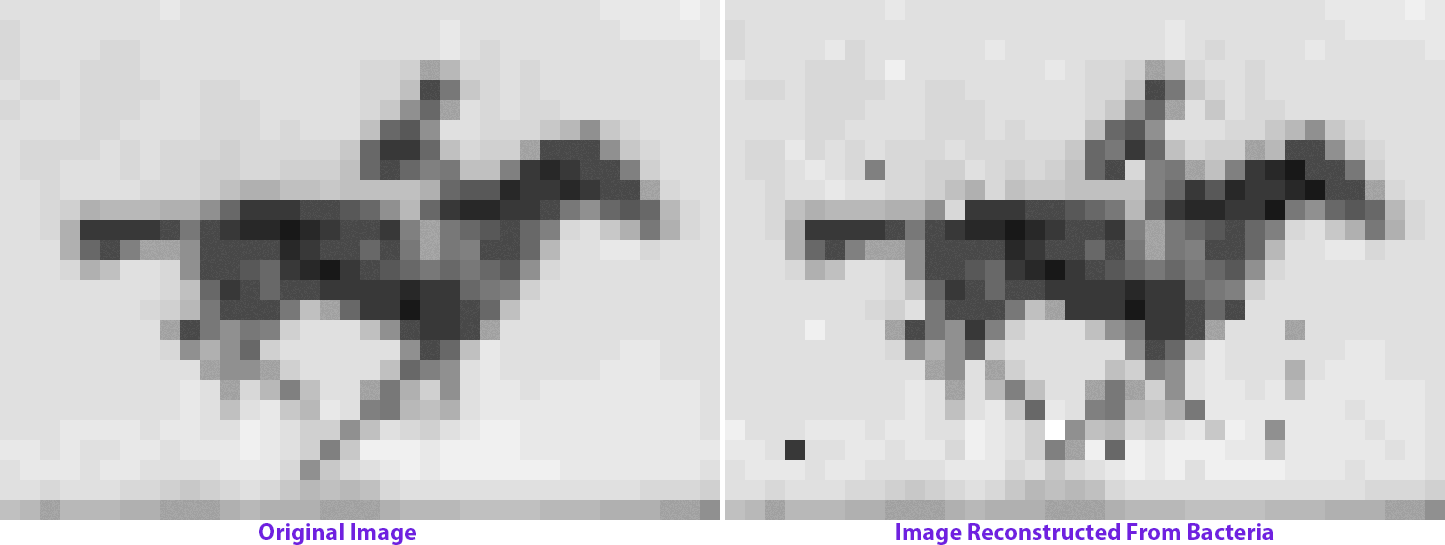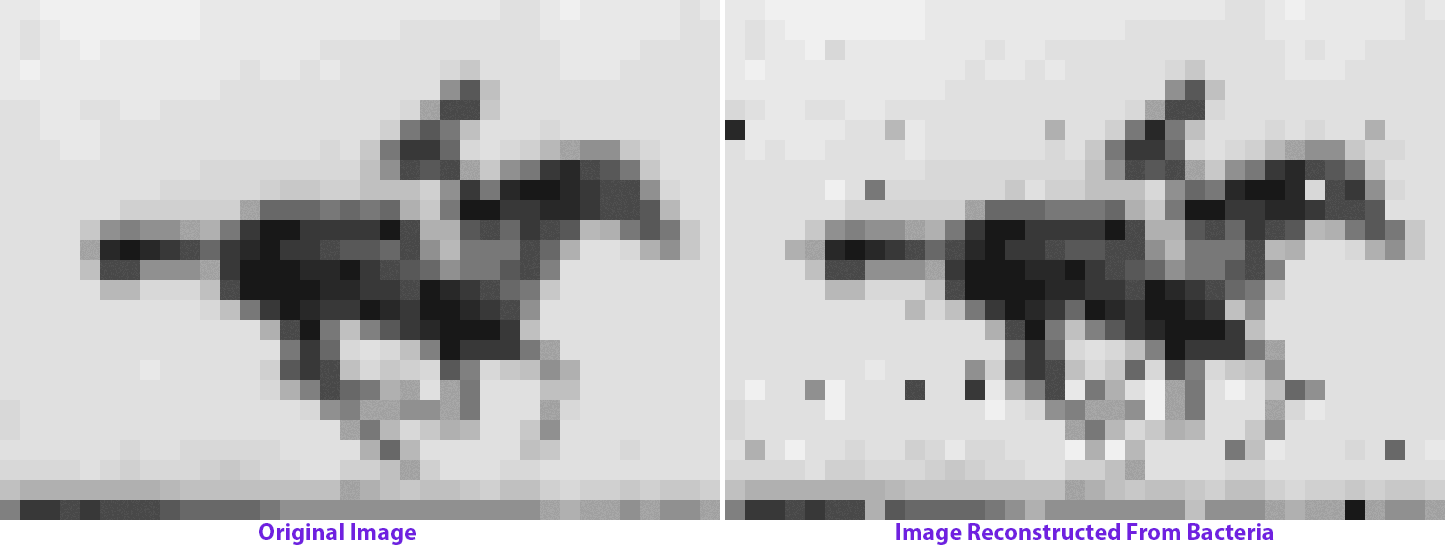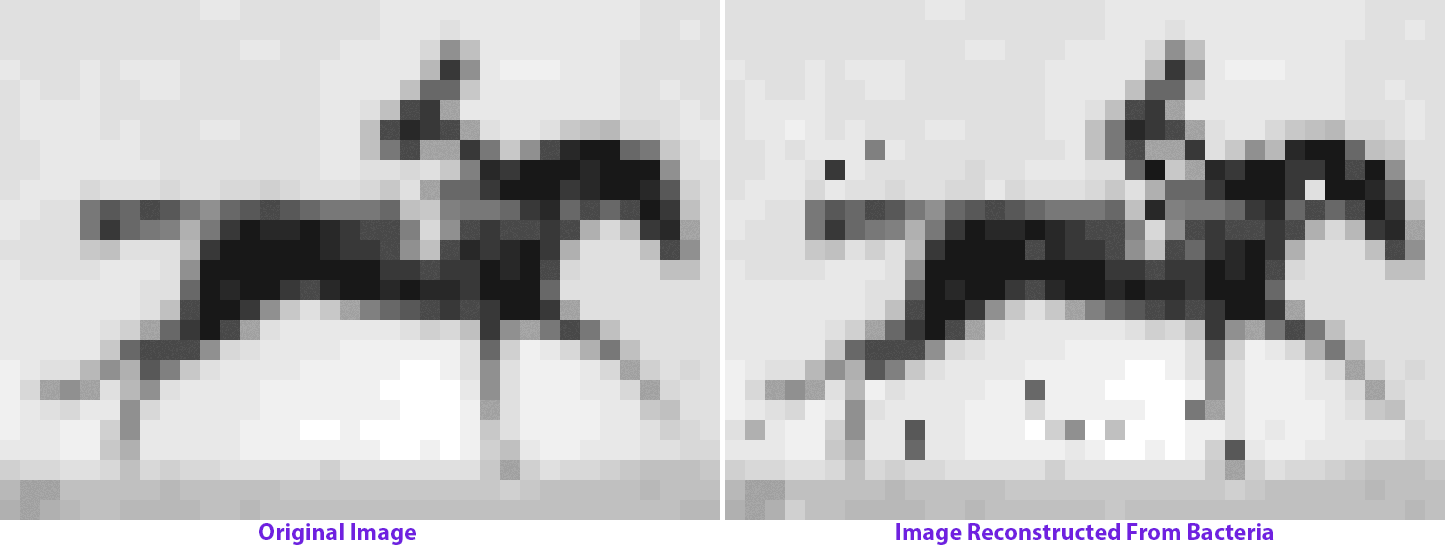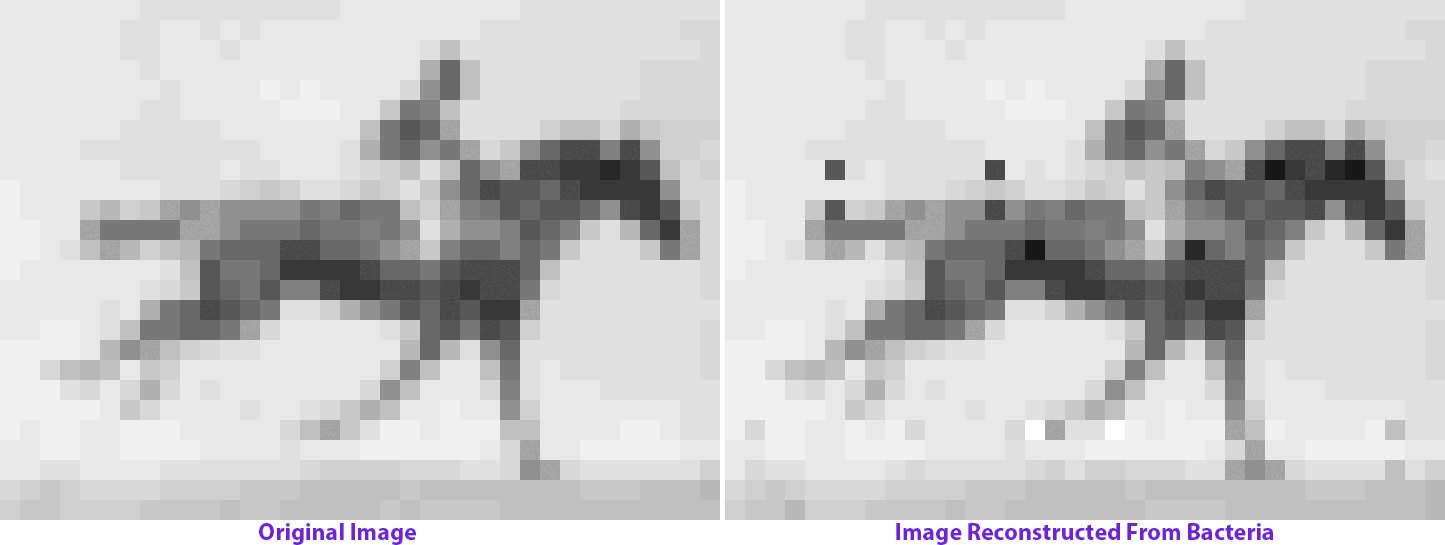
The molecular recorder is a CRISPR-Cas-based system that works inside of a cell and logs biological data by making modifications to the cell’s genome. So it changes the genome over time, and if those changes are written one after the other into the genome, then it can not only capture data, but it can also capture a time course of events. We were able to record sequences of DNA that I delivered to cells over time and reconstruct that time course at a later point by sequencing the cells. That basically forms the backend, or framework, of a data-acquisition device that functions inside of living cells. It captures pieces of DNA, puts them in a cell’s genome and keeps the order with respect to time.
How are you extending your postdoctoral work to the research that you’re doing now as an independent investigator?
My postdoc work really just began to build technology and establish a proof-of-concept stage for something that could be applied. Now we’re applying it to record biological data and understand the cellular processes that guide the development of complex tissues like the brain. We want to be able to record gene expression over time in living cells and push [this approach] from where it started in bacteria into mammalian cells and eventually into the developing brain.
“It’s appealing to me to be able to go in and try to make fundamental insights that can potentially one day make people’s lives better.”
How did you become interested in autism research?
I’ve been working in and around the field of autism for a long time. I have a real desire to make a contribution to the understanding of neurodevelopmental disorders, which can be so devastating and affect people throughout their life. These are incredibly complex disorders. Autism and neurodevelopmental disorders in general don’t have a clear easy answer at this point, and we need to be able to have a better understanding of the fundamental biology of the brain and especially the developing brain. As a researcher, it’s appealing to me to be able to go in and try to make fundamental insights that can potentially one day make people’s lives better.
Do you think that the Bridge to Independence Award helped distinguish you from other candidates during your search for a faculty position?
I think it absolutely did. It was evidence that I could go out and bring in some funding, but more than that, I think that because SFARI and the Simons Foundation are well-known and respected in the field, it really indicated to potential future colleagues that my science was something exciting to these communities and that my commitment to understanding these disorders was also something that was recognized.
What challenges have you faced in setting up your own laboratory? And how has your SFARI funding helped in this transition?
Setting up a new lab is an incredible experience; it’s not the easiest thing to do, but it is also really exciting. As a Principal Investigator, there are a whole host of other things that you’re also responsible for, in addition to research, such as mentoring the people in your lab, making sure that the lab is running and funded, managing budgets and also interacting with your colleagues to build connections and find collaborations. At the same time, you’re still pushing the science forward — I’m still at the bench. It’s a lot to do and jump into all at once. The SFARI funding definitely has eased that transition, by providing flexible funds that I was able to use to really launch [my lab]. I can bring students in and we can just immediately start doing experiments.
“Having this funding allows us to be able to do some additional experiments that could really change the way that we will do our science forever in this lab. ”
One of the most incredible, but also daunting, aspects of starting a new lab is that we’re trying to set out onto a path that is unique for the lab, which means that we’d like to be able to take some risks and try some things that aren’t absolutely guaranteed to work but, if they do work, would really be an inflection point in the field. Having this funding allows us to be able to do some additional experiments that could really change the way that we will do our science forever in this lab.
What advice would you give to fellows who consider applying for the Bridge to Independence Award and to other awardees who are currently looking for a faculty position?
My advice to both of them would be somewhat similar. There’s a lot to manage as you go from a postdoc position into an independent position, but the biggest thing that can help is to really do something that you love and that is inspiring to you, and then communicate that to foundations that may fund you, like SFARI, and to institutions that may give you a job. So what you’re bringing forward is really not an attempt to find a job somewhere, but to broadcast to the world what it is that you’d like to do and then identify the right people to help you make that happen.
Your research is pioneering in many respects and has potential applications for different fields of biology and neuroscience. In what ways do you think it would help advance autism research?
In the field of autism, we now have a much better understanding of the diversity of potential mutations that can lead to the disorder, but those lists of mutations haven’t yet given us a cellular mechanism of the disorder. We need a stronger framework for the understanding of the developing brain in order to interpret these lists of potentially causative mutations, so we can start to have a predictive model of what’s going on in the developing brain and say, “Well, if a given protein is mutated, how might that affect the type of cells that exist in the brain, the type of circuits, and how might that shape the entire system?”
What we want to do is to really firm up all those rules. We’d like to be able to build these rule-based models for how you get cell types and how you then assemble those cell types into the intrinsic circuits of the brain. With that model in hand, we will be much, much better positioned to interpret all this incredible genomic data and to try to see what the general patterns that might be going on are and that might converge onto the cellular phenotype.
What aspects of autism research do you find most challenging and what aspects do you find most exciting to work on?
Studying the brain is challenging because it’s incredibly diverse and incredibly complicated. That’s even more difficult in autism, which occurs in the context of a tissue that’s changing over time. I think all of that complexity is probably the biggest challenge in understanding what the root of the disorder is. But it’s also what makes it most exciting, because you have to take new approaches to try to understand how we’re going to manage this complexity and how we’re going to build new tools that are designed to really probe the system to give us access to that data that we don’t yet have.


