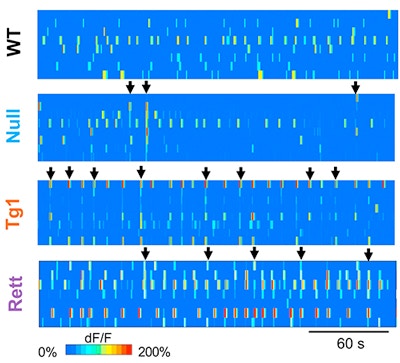
Rett syndrome and MeCP2 duplication syndrome are caused by loss- and gain-of-function mutations in MeCP2, respectively. The disorders have overlapping neurological symptoms, including autism features, seizures, motor impairments and intellectual disability, yet they show inverse patterns of gene expression and synaptic regulation. SFARI Investigators Huda Zoghbi and Stelios Smirnakis investigated how these opposing molecular deficits lead to similar behavioral phenotypes. Using a variety of mouse models of these two disorders, combined with two-photon calcium imaging and electrophysiology, the researchers found that both models exhibit hippocampal circuit dysfunction that results in neuronal hypersynchrony. Chronic forniceal deep brain stimulation, previously shown to rescue behavioral deficits in Rett syndrome mice (Hao et al., 2015), reversed the abnormal network activity. Interestingly, hypersynchrony in the neocortex and increased correlated activity in the prefrontal cortex have been observed in two other mouse models of autism spectrum disorders (Gonçalves et al., 2013; Luongo et al., 2016). Considered together, these data suggest that neural circuit hypersynchrony may be a core feature of autism spectrum disorders and a potential target for therapeutic intervention.
Reference(s)
Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a Rett syndrome mouse model.
Lu H., Ash R.T., He L., Kee S.E., Wang W., Yu D., Hao S., Meng X., Ure K., Ito-Ishida A., Tang B., Sun Y., Ji D., Tang J., Arenkiel B.R., Smirnakis S., Zoghbi H.


