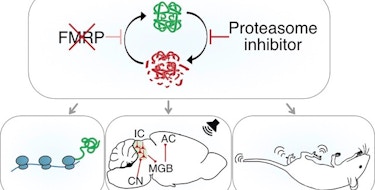
A functioning nervous system needs synapses capable of strengthening or weakening their connections between neurons in response to changes in activity, a cellular process known as synaptic plasticity. Translation of mRNAs into proteins at synapses plays a fundamental role in supporting synaptic plasticity, and mutations in genes that regulate mRNA translation are associated with an increased risk for autism spectrum disorder (ASD) (Louros and Osterweil, J. Neurochem., 2016). One example of this is fragile X syndrome (FXS), a common heritable cause of ASD, caused by mutations in the FMR1 gene and a loss of the RNA-binding protein that it encodes, FMRP.
Initial research on FMRP used in vitro models and artificial reporters and found that FMRP may suppress translation of mRNA into proteins (Laggerbauer et al., Hum. Mol. Genet., 2001; Li et al., Nucleic Acids Res., 2001) and that a loss of FMRP could result in a harmful build-up of proteins at the synapse (Darnell et al., Cell, 2011; Das Sharma et al., Cell Rep., 2019), which fit with data that translation is globally increased in the absence of FMRP (Gross et al., J. Neurosci., 2010; Osterweil et al. J. Neurosci., 2010). However, contrary to this prevailing evidence, a 2018 study by SFARI investigator Ethan Greenblatt which measured translation in vivo in fruit flies (Drosophila), found that FMRP in fact activates translation of its targets, particularly of large mRNAs that are longer in length than the average-sized mRNAs (Greenblatt and Spradling, Science, 2018). Two recent studies — one led by Greenblatt (Flanagan et al., Genetics, 2022) and another led by Emily Osterweil (Seo et al., Nat. Commun., 2022) — more precisely clarify FMRP’s role in regulating the translation of mRNA and how altered translation in neurons could disrupt synaptic function in models of FXS.
Supported in part by the Simons Initiative for the Developing Brain (SIDB), Osterweil and her colleagues found an imbalance (rather than a general overproduction) in the translation of long versus short mRNAs in hippocampal neurons of juvenile mice missing FMR1 (Seo et al., Nat. Commun., 2022). This imbalance may be driven by excessive translation of ribosomal proteins or by the underproduction of large proteins. Greenblatt, supported in part by a SFARI Bridge to Independence Award, similarly showed with his colleagues that FMRP not only activates the translation of large proteins encoded by long mRNAs, but also that this role of FMRP is evolutionarily conserved across species, as it was observed both in Drosophila egg cells and the mammalian (mouse) cortex (Flanagan et al., Genetics, 2022). Together, the independent findings by Greenblatt and Osterweil set the stage for a rethinking of FMRP’s role in mRNA translation. This new model offers future studies an alternate route to explore how disruptions in synaptic plasticity due to FMRP deficiencies could lead to changes in cognitive functioning characteristic of FXS and ASD.
Reference(s)
FMRP-dependent production of large dosage-sensitive proteins is highly conserved.
Flanagan K., Baradaran-Heravi A., Yin Q., Dao Duc K., Spradling A.C., Greenblatt E.
Excess ribosomal protein production unbalances translation in a model of fragile X syndrome.
Seo S.S., Louros S.R., Anstey N., Gonzalez-Lozano M.A., Harper C.B., Verity N.C., Dando O., Thomson S.R., Darnell J.C., Kind P., Li K.W., Osterweil E.K.