SFARI is funding a number of efforts to determine if at least some of the genes implicated in autism spectrum disorder (ASD) risk via de novo mutation converge on particular biological pathways or processes. A new study identifies presynaptic homeostatic plasticity (PHP) as one possible point of biological convergence of ASD risk genes, which seem to sensitize the organism to the effects of a group of common modifiers of PHP.
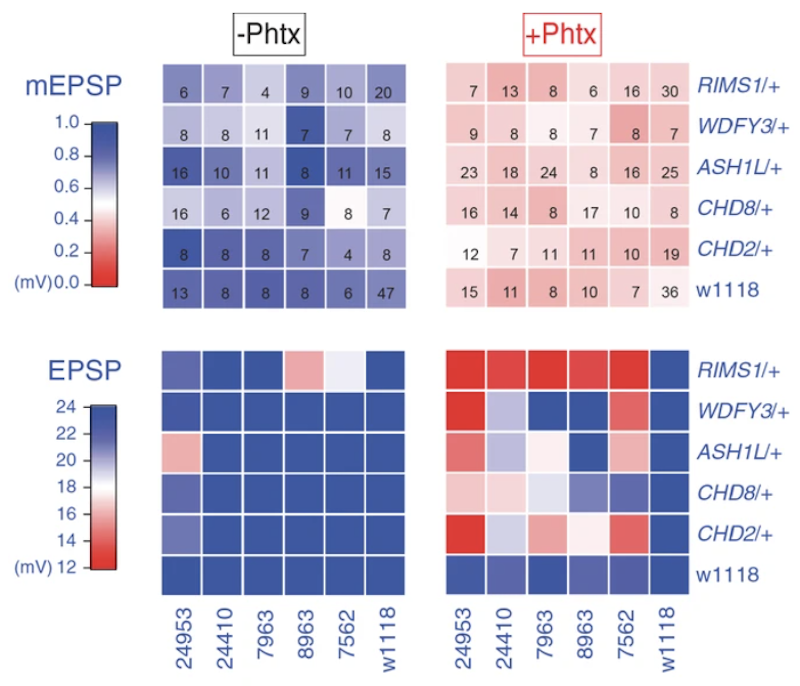
The work was supported in part by a Pilot Award to SFARI Investigator Graeme Davis. For their assay of PHP, the authors examined the Drosophila neuromuscular junction, at which PHP can be induced by the application of sub-blocking concentrations of a postsynaptic glutamate receptor antagonist. Heterozygous loss-of-function mutations in the orthologs of five different ASD risk genes—RIMS1, CHD8, CHD2, WDFY3 and ASH1L—showed no effect on PHP, but compound heterozygous mutations in RIMS1 and either CHD8, CHD2 or ASH1L significantly impaired PHP. A forward genetic screen using small chromosomal deficiencies covering about 6,000 genes in the Drosophila genome further identified a surprisingly large number of double-mutant combinations affecting PHP. Fine mapping of two of these deficiency strains revealed PDPK1 and PPP2R5D as two of the genes conferring this interaction effect on PHP. Interestingly, the CHD8-PPP2R5D double-mutant interaction showed altered presynaptic membrane trafficking as a possible cellular mechanism underlying the diminished PHP.
Of note, neither the ultrastructural differences seen in the CHD8-PPP2R5D double-mutant, nor the gene expression analyses, nominated a mechanism for the altered PHP that was common to a significant number of gene pairs. These findings suggest that the role of ASD risk gene mutations in sensitizing neurons to altered PHP is likely to be complex. The authors also suggest that these interaction-based mechanisms may underlie some degree of resilience to ASD in unaffected individuals who carry mutations that are typically associated with a diagnosis.
Reference(s)
Homeostatic plasticity fails at the intersection of autism-gene mutations and a novel class of common genetic modifiers.
Genç Ö., An J.-Y., Fetter R.D., Kulik Y., Zunino G., Sanders S., Davis G.


