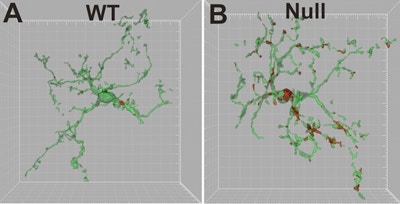
Rett syndrome (RTT), an X-linked neurodevelopmental disorder, is caused by mutations in MeCP2. Microglia, the resident macrophages in the central nervous system (CNS), have been implicated in RTT pathogenesis, though it remains unclear whether and how microglia specifically contribute to the syndrome. SFARI Investigator Beth Stevens previously showed that microglia play a role in synaptic circuit remodeling in the healthy brain, pruning synapses by phagocytosing weaker presynaptic inputs (Schafer et al., 2012). In the current study, Stevens and her colleagues show that microglia-mediated synaptic remodeling is abnormal in MeCP2 null mice, with microglia excessively eliminating presynaptic inputs at end stages of disease progression. However, excessive synaptic pruning is unaffected by the level of MeCP2 expression in microglia. These data suggest that microglia respond secondarily, targeting circuits and synapses that have been previously weakened and rendered vulnerable by loss of MeCP2 in other CNS cell types.
Reference(s)
Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression.
Schafer D., Heller C.T., Gunner G., Heller M., Gordon C., Hammond T., Wolf Y., Jung S., Stevens B.


