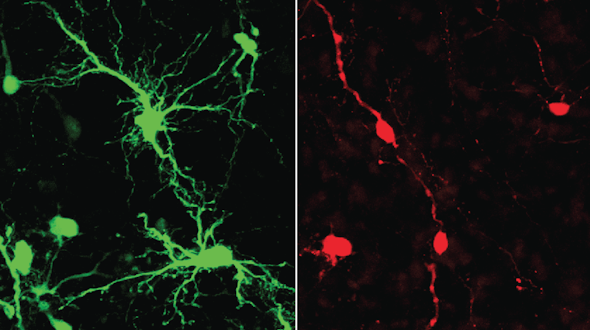
SFARI-funded research finds that RAPGEF4 is a key molecular regulator orchestrating prefrontal cortical maturation in primates.
Highlights of SFARI-funded papers, selected by the SFARI science team.

SFARI-funded research finds that RAPGEF4 is a key molecular regulator orchestrating prefrontal cortical maturation in primates.
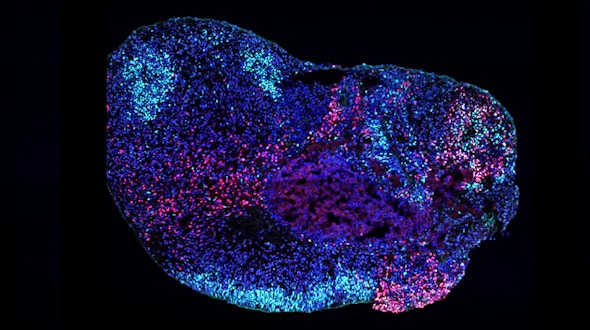
SFARI-funded research finds that demonstrates that SCN8A mutations underlying DEE-13 disrupt both cortical and hippocampal networks, but in distinct ways.



Large-scale genetic studies have now uncovered more than 200 genes that can be linked to neurodevelopmental disorders (NDDs) or autism. Many of those genes are implicated through gene-damaging heterozygous de novo mutations that result in haploinsufficiency of the affected gene. Identifying such genes opens the door for the development of gene-specific therapeutics like gene replacement therapy. However, gene replacement therapy comes with many challenges, one of them being the risk of overexpression. Therefore, therapeutics that could result in the upregulation of the remaining healthy allele and do not interfere with endogenous regulatory mechanisms of gene expression would be ideal.

Social behavior is a complex and dynamic process shaped by movement, coordination and physical touch. A new study in Cell written by members of SFARI's Autism Rat Consortium introduces s-DANNCE, a machine-learning system that can map the fine-scale movements of freely interacting rats in three dimensions. By applying s-DANNCE to seven genetic rat models of autism, researchers have uncovered distinct social phenotypes, offering new insights into the diversity of autism-related behaviors.
Two studies by different research groups — one led by Flora Vaccarino and the other by Juergen Knoblich — used brain organoids derived from pluripotent stem cells of people with autism and showed how transcriptional alterations affecting certain cell types during human brain development could contribute to the early emergence of ASD.
In a mouse model of fragile X syndrome, Emily Osterweil and her colleagues show that excessive protein synthesis drives a pathological compensatory rise in protein degradation (by the ubiquitin proteasome system), which can be targeted to correct various phenotypes including audiogenic seizures.

A study by Caroline Robertson and her colleagues found that reduced social attention was not a static omnipresent characteristic of autism; rather, it was magnified only under certain real-world conditions where sensory processing demands were high.

Elise Robinson and colleagues identified a large genomic region — chromosome 16p — where a rare 16p11.2 variant associated with autism functionally converges with common polygenic variation across 16p. Both rare and common genetic variation at 16p decreased expression of neuronally expressed genes, with relevance for increasing autism risk.