TEK IMAGE/Science Source
The Innovative Medicines Initiative (IMI), a European public-private initiative, recently awarded a €115 million five-year grant for autism research, entitled “Autism Innovative Medicine Studies-2-Trials” (AIMS-2-Trials).
Led by Declan Murphy (professor of psychiatry and brain maturation, King’s College London) and Will Spooren (section head, Behavioural Pharmacology and Preclinical Imaging, F. Hoffmann-La Roche), AIMS-2-Trials brings together 48 partners across 14 countries from academia, industry and nonprofit foundations, including the Simons Foundation Autism Research Initiative (SFARI). The aim of this large-scale, international collaboration is to advance the understanding of autism spectrum disorder (ASD) pathophysiology, develop biomarkers and test treatments to improve health outcomes for people living with the disorder.
AIMS-2-Trials will build on the results of the European Autism Interventions – A Multicentre Study for Developing New Medications (EU-AIMS), a five-year grant that was previously awarded by the IMI to investigate ASD mechanisms and opportunities for translational research.
Using a combination of animal experimental models, clinical cohorts and human-derived induced pluripotent stem cells, AIMS-2-Trials will identify and validate ASD biomarkers (including those generated by EU-AIMS) (Figure 1), further investigate the cellular-, circuit- and system-level abnormalities of the condition, and explore genetic and environmental risk factors. By adopting a precision medicine approach, the project will also stratify the ASD population and develop and test targeted interventions.

“Many individuals on the spectrum face extremely poor health outcomes, yet autism research receives far less investment than other conditions that also limit life expectancy and quality of life, such as cancer or dementia,” said Murphy in the press release of the study. “This grant will allow us to bridge the gap between basic biology and the clinic by offering personalized approaches that address problems that really impact people’s lives.”
To this end, AIMS-2-Trials will form the first European-wide autism clinical trials network and assess the efficacy of novel compounds and repurposed drugs. One of these drugs includes arbaclofen, a GABAB receptor agonist that has shown promising preliminary results in a subset of people in a previous clinical trial1, although its efficacy remains to be fully ascertained. Arbaclofen for the AIMS-2-Trials study will be supplied by Clinical Research Associates, L.L.C (CRA), an affiliate of the Simons Foundation.
“CRA is very pleased to provide arbaclofen for the AIMS-2-Trials study,” says Paul Wang, Deputy Director of CRA. “Previous research has shown that arbaclofen may be able to support social function in ASD, but additional studies are certainly needed. The design of the current study draws on lessons learned in previous trials and will assess both clinical and biomarker endpoints.”
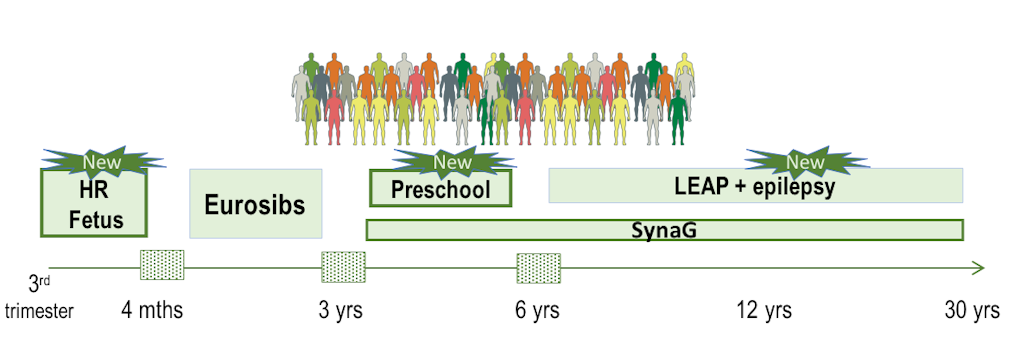
AIMS-2-Trials also plans to generate the largest autism longitudinal cohort to date. In addition to the European Babysibs Autism Research Network (Eurosibs, high-risk infants through 3 years old children) and Longitudinal European Autism Project (LEAP, 6–30 years old) funded by EU-AIMS2-5, this study will include new cohorts. These cohorts include pregnant mothers whose babies are at high familial risk for autism, preschoolers (age 3–6 years) and individuals with monogenic forms of ASD. Very large cohorts of children in South Africa who were exposed to high rates of maternal ill health will also be followed up. In addition, AIMS-2-Trials will address co-morbidities; individuals with autism and epilepsy will be recruited, together with previous participants of the LEAP cohort, to participate in a component of the study examining epileptic signatures in ASD (Figure 2). Adding these new groups will help researchers track the condition from its inception during prenatal life through childhood and into adulthood.
‘Through the IMI, EU funding matched in-kind contributions from nonprofit foundations and industry representatives; SFARI, Autism Speaks and Autistica contributed nearly €55.5 million and €2.5 million was provided by the European Federation of Pharmaceutical Industries and Associations.
“International coordinated clinical research including epidemiology, natural history, stratification, biomarkers, outcome measures and clinical trials will allow us all to move forward faster, and we are thrilled to see autism as a major focus of the IMI,” says Wendy Chung, Director of Clinical Research at SFARI and the Kennedy Family Professor of Pediatrics in Medicine at Columbia University.


